Nickel-Catalyzed Cross-Electrophile Coupling of Aryl Phosphates with Aryl Bromides
Jing-Ao Ren,a Xue Chen,a Chao Gui,a Chengping Miao,b Xue-Qiang Chu,a Hao Xu,*a Xiaocong Zhou,*b Mengtao Ma,c and Zhi-Liang Shen*a
a Technical Institute of Fluorochemistry (TIF), Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, China. E-mail: xuhao@njtech.edu.cn; ias_zlshen@njtech.edu.cn.
b College of Biological, Chemical Science and Engineering, Jiaxing University, 118 Jiahang Road, Jiaxing 314001, China. E-mail: xczhou@zjxu.edu.cn.
c College of Science, Nanjing Forestry University, Nanjing 210037, China.
Abstract: A step-economical and operationally simple nickel-catalyzed cross-electrophile coupling of aryl phosphates with aryl bromides through C-O bond cleavage, which precluded the employment of relatively moisture-labile and unreadily available organometallics, was developed. The reaction proceeded smoothly in the presence of magnesium turnings and lithium chloride in THF to afford the corresponding biaryls in moderate to good yields with reasonable functionality tolerance.
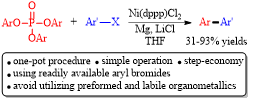
Advanced Synthesis & Catalysis 2023, 365, 2511-2515. (VIP paper. Impact factor: 5.4)
论文链接:https://onlinelibrary.wiley.com/doi/10.1002/adsc.202300663
