Nickel-Catalyzed Direct Cross-Coupling of Diaryl Sulfoxide with Aryl Bromide
W.-X. Li,a B.-W. Yang,a X. Ying,a Z.-W. Zhang,a X.-Q. Chu,*a X. Zhou,*b M. Ma,c Z.-L. Shen*a
a Technical Institute of Fluorochemistry (TIF), Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, China.
b College of Biological, Chemical Science and Engineering, Jiaxing University, 118 Jiahang Road, Jiaxing 314001, China.
c Jiangsu Provincial Key Lab for the Chemistry and Utilization of Agro-Forest Biomass, College of Chemical Engineering, Nanjing Forestry University, Nanjing 210037, China
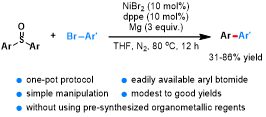
Abstract: The direct cross-couplings of diaryl sulfoxides with aryl bromides via C−S bond cleavage could be readily accomplished using nickel(II) as the catalyst, 1,2-bis(diphenylphosphino)ethane (dppe) as the ligand, and magnesium turnings as the reducing metal in THF, leading to the corresponding biaryls in moderate to good yields. The reaction exhibited a broad substrate scope and could be applied to a gram-scale synthesis. The “one-pot” reaction, which avoids the utility of presynthesized and moisture-labile organometallic compounds, is operationally simple and step-economic.
J. Org. Chem. 2022, DOI: 10.1021/acs.joc.2c01513. (Impact factor: 4.198)
论文链接:https://pubs.acs.org/doi/10.1021/acs.joc.2c01513
