Desulfonylation via Radical Process: Recent Developments in Organic Synthesis
Xue-Qiang Chu,*a Danhua Ge,a Yan-Ying Cui,a Zhi-Liang Shen,*a and Chao-Jun Li*b
a Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, China.
b Department of Chemistry and FQRNT Centre for Green Chemistry and Catalysis, McGill University, Montreal, Quebec H3A 0B8, Canada
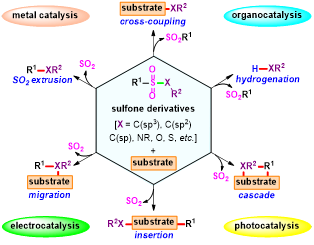
Abstract: As the “chemical chameleon”, sulfonyl-containing compounds and their variants have been merged with various types of reactions for the efficient construction of diverse molecular architectures by taking advantage of their incredible reactive flexibility. Currently, their involvement in radical transformations, in which the sulfonyl group typically acts as a leaving group via selective C–S, N–S, O–S, S–S, and Se–S bond cleavage/functionalization, has facilitated new bond formation strategies which are complementary to classical two-electron cross-couplings via organometallic or ionic intermediates. Considering the great influence and synthetic potential of these novel avenues, we summarize recent advances in this rapidly expanding area by discussing the reaction designs, substrate scopes, mechanistic studies, and their limitations, outlining the state-of-the-art processes involved in radical-mediated desulfonylation and related transformations. With a specific emphasis on their synthetic applications, we believe this review will be useful for medicinal and synthetic organic chemists who are interested in radical chemistry and radical-mediated desulfonylation in particular.
Chem. Rev. 2021, DOI: 10.1021/acs.chemrev.1c00084. (Impact factor: 60.622)
论文链接:https://pubs.acs.org/doi/10.1021/acs.chemrev.1c00084.
