Mechanistic Study of the N‑Quaternized Pyridoxal-Catalyzed Biomimetic Asymmetric Mannich Reaction: Insights into the Origins of Enantioselectivity and Diastereoselectivity
Xianlu Cui‡a, Qianqian Li‡a, Lei Yaoa, Yanshun Maa, Lixiong Zhangb, Chuanbao Zhangc, Lili Zhaoa*
aInstitute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, China. Email: ias_llzhao@njtech.edu.cn
bCollege of Chemical Engineering, State Key Laboratory of Materials-Oriented Chemical Engineering, Nanjing Tech University, Nanjing 211816, China
cCollege of Chemistry and Chemical Engineering, Henan University, Kaifeng 475004, China.
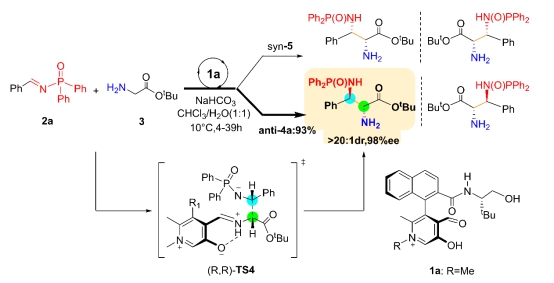
ABSTRACT: Density functional theory (DFT) calculations have been performed to gain insight into the catalytic mechanism of the N-quaternized pyridoxals (i.e., 1a) mediated biomimetic asymmetric Mannich reaction of tert-butyl glycinate 3 with N-diphenylphosphinyl imine 2a to give the diamino acid ester 4a in high yield with excellent enantiomeric and diastereomeric selectivity (Science 2018, 360, 1438). The study reveals that the whole catalysis can be characterized via three stages: (i) the catalyst 1a react with the tert-butyl glycinate 3 generate the active carbanion complex IM3. (ii) IM3 then reacts with the N-diphenylphosphinyl imine 2a giving the imine intermediate IM8. (iii) IM8 undergoes hydrolysis to give the final product anti-4a and regenerate the catalyst 1a for the next catalytic cycle. Each stage is kinetically and thermodynamically feasible for experimental realization. The hydrolysis step in the stage III is predicted to be the rate-determining step (RDS) during the whole catalytic cycle. Furthermore, the origins of the enantioselectivity and diastereoselectivity for the target reaction, as well as the deactivation of the catalyst 1b, are also discussed.
J. Org. Chem. 2021, 86, 9, 6592–6599 影响因子:4.335
论文链接:https://pubs.acs.org/doi/10.1021/acs.joc.1c00381
