2H‑Azirines as Potential Bifunctional Chemical Linkers of Cysteine Residues in Bioconjugate Technology
Yang Chen, a Wenjie Yang, a Jiamin Wu, a Wangbin Sun, a Teck-Peng Loh,* ab and Yaojia Jiang* a
a Institute of Advanced Synthesis, Nanjing Tech University, Nanjing 211816, P. R. China.
b Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 637616, Singapore.
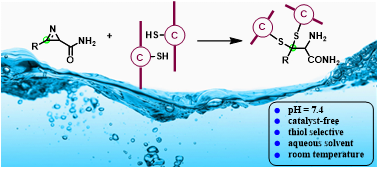
Abstract: 2H-Azirine-2-caboxamides have been designed to perform as a new type of bifunctional thiol linker under very mild reaction conditions. The cleavage of a C−N double bond of 2Hazirine furnishes an amino amide functional group in situ through a thiol addition and ring-opening process. It works with a broad scope of thiols and 2H-azirines in the absence of any catalysts at room temperature. Cysteine-containing peptides have also been demonstrated to work efficiently in a completely water solution.
Org. Lett. DOI: 10.1021/acs.orglett.0c00415. (2019年影响因子:6.555)
文章链接: https://pubs.acs.org/doi/10.1021/acs.orglett.0c00415.
