Reciprocal-Activation Strategy for Allylic Sulfination with Unacti-vated Allylic Alcohols
Peizhong Xie*a, Zuolian Suna, Shuangshuang Lia, Xinying Caia, Ju Qiua, Weishan Fua, Cuiqing Gaob, Shisheng Wuc, Xiaobo Yanga, and Teck-Peng Loh*ad
a School of Chemistry and Molecular Engineering, Institute of Advanced Synthesis, Nanjing Tech University, Nanjing 211816, P. R. China.
b Co-Innovation Center for the Sustainable Forestry in Southern China, College of Forestry, Nanjing Forestry University, Nanjing, 210037, China.
c CNPC Northeast Refining & Chemical Engineering Co., Ltd Shenyang Company, Shengyang110167, P. R. China.
d Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371.
Abstract: A reciprocal-activation strategy for allylic sulfination with unactivated allylic alcohols was developed. In this reaction, the hydrogen bond interaction between allylic alcohols and sulfinic acids allowed for reciprocal activation, which enabled a dehydrative cross-coupling process to occur under mild reaction conditions. This reaction worked in an environmentally friendly manner, yielding water as the only byproduct. A variety of allylic sulfones could be obtained in good to excellent yields with wide functional group tolerance. In gram scale reactions, allylic sulfones could be conveniently isolated in high yield by filtration.
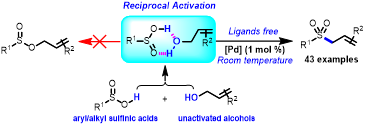
Org. Lett, 2020, DOI: 10.1021/acs.orglett.0c01747(影响因子:6.555)
论文链接:https://pubs.acs.org/doi/10.1021/acs.orglett.0c01747
